Lye in Soap: Dangerous or Necessary?
When I told my mom that I was going to start selling soaps made with lye , her first reaction was one of surprise. “Isn’t that really hard on your skin?”
My own mother, readers, was wary of lye. And let’s be honest— “lye” isn’t the most comforting word in the world. It sounds harsh, industrial, and maybe even toxic (see my first blog post to read about toxic chemicals).
When people first learn that lye is used to make soap, but they often have that same reaction: “Wait—lye? Isn’t that really dangerous?”
It’s a great question and one I love answering. Let’s break it down.
What is Lye, Exactly?
Lye is the common name for the compound sodium hydroxide (NaOH). It is an alkaline compound used in many applications including traditional soap making. It is known for being caustic meaning that it will cause chemical burns if not handled correctly.
So, yes, lye can be dangerous. To handle it, you need gloves, probably an apron, goggles, and a lot of care and respect. But just like vinegar, bleach, or even boiling water, the danger depends on context and concentration.
Why is Lye Used in Soap Making?
The short answer: You can’t make soap without lye.
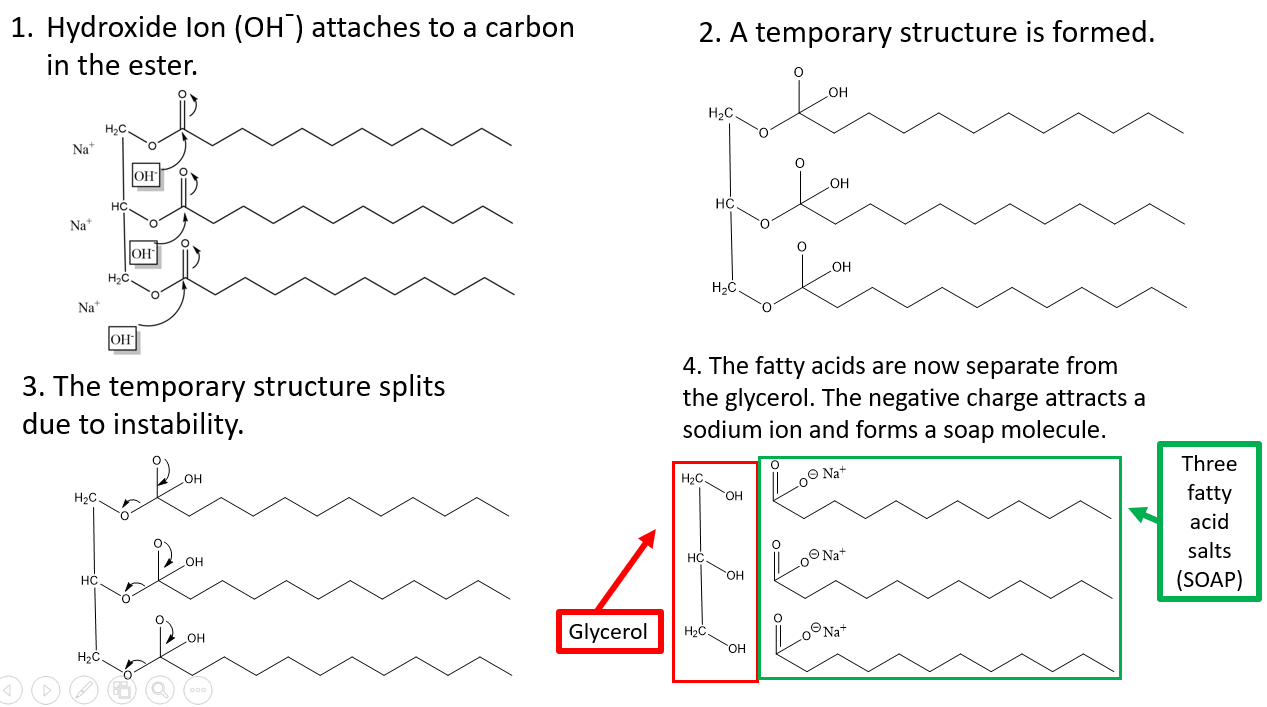
Lye is essential to a process called saponification— the chemical reaction that creates soap. During saponification, lye reacts with the triglycerides that are in oils (Curious about triglycerides? See my post on Fatty Acids & Glycerol).
Triglycerides consist of three fatty acids and a glycerol. They are connected together with an oxygen atom creating a structure that chemists call an ester functional group.
When Lye, or sodium hydroxide, is dissolved in water. It splits into a sodium (Na) ion with a positive charge and a hydroxide (OH) ion with a negative charge. These ions react with the ester functional group in the triglyceride molecule to form:
Soap Molecules (which clean your skin), and
Glycerin (a humectant that helps to moisturize your skin).
Without lye, you don’t get soap— you just have oil.
Is Lye Dangerous in Soap?
This is the part that most people don’t realize (my mother included): when soap is made properly, there is no lye left in the final bar.
Once the oils and lye have reacted, the lye is completely transformed. It’s gone. Chemically speaking, it no longer exists. Instead, it is now glycerin and soap.
Our Commitment to Safe, Science-Backed Skincare
At The Clean Chemist, we embrace the power of clean chemistry. We don’t cut corners or use vague language. We believe our customers deserve to understand what goes into their products—and why.
We use lye with precision and purpose, and we balance it with nourishing oils and butters that leave your skin feeling clean, hydrated, and healthy. And of course, we test every batch for safety and pH before it ever touches your skin.
At The Clean Chemist, we formulate every batch with care. Our recipes are “superfatted,” which means we leave a small extra amount of oil to ensure all the lye is fully used up—so there’s zero risk of leftover lye in our bars.
We also cure our soaps to ensure they’re gentle, long-lasting, and skin-safe.
Final Thoughts: Lye Is Necessary—But Not Dangerous in Soap
Lye is essential to making real soap. It’s part of what transforms natural ingredients into a powerful, cleansing, and moisturizing bar. And while it should be handled carefully during the process, it’s completely safe in the final product.
So the next time you hear that a soap contains lye, you can smile—and say, “Of course it does. That’s how you make soap.”